ORIGINAL PAPER
Exploring the Mechanisms of Anti-Aβ42 Aggregation Activity of Walnut-derived Peptides using Transcriptomics and Proteomics in vitro
1
School of Food Science and Engineering, South China University of Technology, China
Submission date: 2021-11-19
Final revision date: 2021-12-09
Acceptance date: 2021-12-14
Online publication date: 2022-01-11
Publication date: 2022-02-01
Corresponding author
Jiaoyan Ren
School of Food Science and Engineering, South China University of Technology, Wushan, 510641, Guangzhou, China
School of Food Science and Engineering, South China University of Technology, Wushan, 510641, Guangzhou, China
eFood 2021;2(5):247-258
KEYWORDS
TOPICS
ABSTRACT
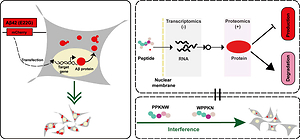
Inhibiting β-amyloid (Aβ) aggregation is of significance in finding potential candidates for Alzheimer’s disease (AD) treatment. Accumulating evidence suggests that nutrition is important for improving cognition and reducing AD risk. Walnut has been widely used as a functional food for brain health; however the underlying mechanisms remain unknown. Here, we investigated the molecular level alteration in Arctic mutant Aβ42 induced aggregation cell model by RNA-seq and iTRAQ approaches after walnut-derived peptides Pro-Pro-Lys-Asn-Trp (PW5) and Trp-Pro-Pro-Lys-Asn (WN5) interventions. PW5 or WN5 could significantly decrease abnormal Aβ42 aggregates. However, resultant alterations in transcriptome (substantially unchanged) were inconsistent with proteomic data (marked change). Proteomic analysis revealed 184 and 194 differentially expressed proteins unique to PW5 and WN5 treatment, respectively, for inhibiting Aβ42 protein production or increasing protein degradation via the mismatch repair pathways. Our study provides new insights into the effectiveness of food-derived peptides for anti-Aβ42 aggregation in AD.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.